Microscopy and Plasmolysis
ABLE 2019 Major Workshop
This version of our student labs has been modified for the 'Using Foldscopes™ for Offline Exploration in Online Biology Courses' major workshop at ABLE 2019. We have combined the Foldscope™ portions of two separate, sequential labs ('Microscopy' and 'Osmosis and Diffusion'), and omitted the other exercises. If you have attended the workshop and have already prepared and posted photos of your red onion specimen, take the following steps:
(1) Download the student questions document from the Handouts box in the sidebar, at left. [A hard copy was also provided during the workshop.]
(2) Skip to the Measuring What You See: Viewing Magnification, Field of View, and Specimen Size (p. 7) and Observing Plasmolysis (p. 19) pages of this lab.
If you would like us to include your ideas for using Foldscopes™ in the revised manuscript for this workshop, please fill out this form. Short- and long-form evaluations of the workshop itself should be completed during the conference, at the direction of the ABLE 2019 organizers.
BIOL 1020 Student Instructions
The full set of learning objectives and required tools and equipment for both labs are provided here for context; those directly related to the Foldscope™ activities are highlighted.
Microscopy Lab Learning Objectives
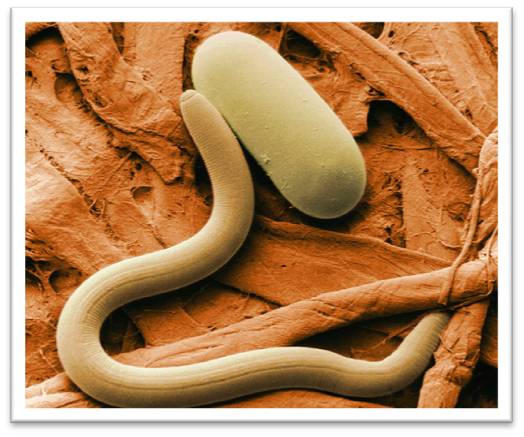
In this lab, we'll work with two microscopes and some images to explore concepts in microscopy. Working with microscopes requires two sets of skills: (1) manipulating the instrument and specimens and (2) interpreting the resulting images. We'll do a bit of both!
After completing this activity and the relevant readings from the textbook (Reece et al., 2018; Chapter 6), you will be able to manipulate a virtual compound light microscope and interpret the resulting micrographs , as well as to interpret representative images produced by electron microscopes. Specifically, you will be able to:
, as well as to interpret representative images produced by electron microscopes. Specifically, you will be able to:
1. Identify the structural components of a basic light microscope.
2. Describe the correct procedure for placing a slide on a light microscope stage, illuminating it appropriately, and bringing the specimen into focus.
3. Recognize and distinguish among images produced by a light microscope, a scanning electron microscope, and a transmission electron microscope.
4. Distinguish between viewing magnification and image magnification by working with images and answering questions that require you to apply your knowledge of these terms.
5. Use field of view, scale bars, and an ocular micrometer to estimate the actual size of a specimen or of specific features of a specimen.
6. From micrographs, identify cell structures and organelles by their appearance, relative size and position within the cell, and/or clues about function.
7. Assemble a portable microscope, collect and prepare specimens, and capture and share images of what you find.
Osmosis and Diffusion Lab Learning Objectives
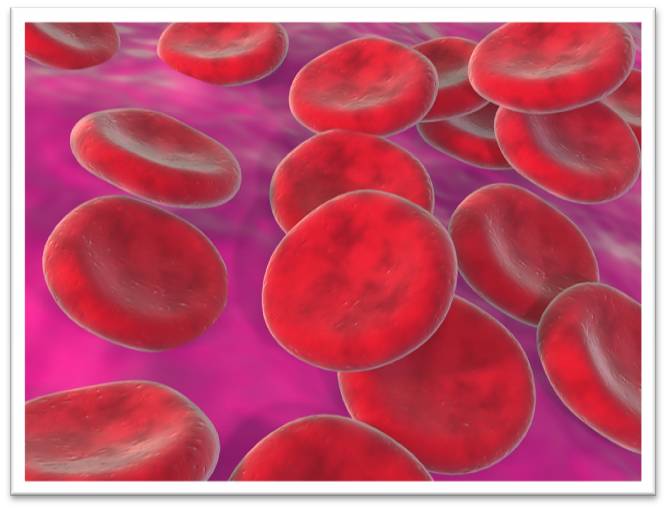
This lab entails studying well known exemplars -- mammalian red blood cells (RBCs), plant cells, and solutions of sodium chloride (NaCl) -- to better understand the processes and physiological significance of diffusion and osmosis. You will work with material that has already been prepared for you, as well as make your own direct microscopic observations with your foldscope.
After completing this activity and the relevant readings from the textbook (Reece et al, 2018; Chapter 7), you will be able to:
1. Define diffusion and osmosis and discuss the physiological significance of these processes at the level of the cell and of the whole organism.
2. Relate molarity to osmolarity and discuss the significance of these terms with respect to biological systems.
3. Define and state the difference between osmolarity and tonicity.
4. Interpret a line graph.
5. Compare and contrast the effects of osmosis on red blood cells and red onion cells, and relate your observations to the structural properties of these cells.
Required Documents, Tools, and Software
- lab questions document: download from the Handouts box in the sidebar [students submit electronic versions of this file for grading via Brightspace]
- a Foldscope™ (hereafter referred to as foldscope) [provided to workshop participants; available for purchase via https://www.foldscope.com ].
- Fiji1 (ImageJ): free download via https://imagej.net/Fiji/Downloads; detailed instructions on its use to follow. If you already have ImageJ, you can use that.
- Padlet: no software or account required; instructions to follow
- a red onion, or piece of red onion - enough to collect several small pieces of epidermis
- tweezers (or your fingernails)
- tap water
- table salt
- two small cups or containers
- a teaspoon or similar small tool for measuring, such as a regular small spoon or a pop bottle cap
- a clean spoon, chopstick, or similar tool to use for stirring
Image Credits
low-temperature scanning electron micrograph of soybean cyst nematode and egg, USDA Agricultural Research Service [Public domain], via Wikimedia Commons
3ds max Red Blood Cell Render by kingdesmond1337 [CC BY-NC-SA2.0] via Flickr
Notes
1Fiji is just ImageJ.
The Osmosis and Diffusion lab was originally developed by David Patriquin and Jennifer Van Dommelen, and subsequently modified by Jennifer Van Dommelen.
The foldscope component of both the Microscopy and the Osmosis and Diffusion labs was developed by Jennifer Van Dommelen and Jacob Fletcher, supported by a Teaching and Learning Enhancement Grant from the Dalhousie Centre for Teaching and Learning.
References
Reece JB, Urry LA, Cain ML, Wasserman SA, Minorsky PV, Jackson RB, Rawle F, Durnford D, Moyes C, Scott K, Walde S. 2018. Campbell Biology. Second Canadian Edition. Hoboken, NJ: Pearson Education Inc.